
A chemical equation is the most beneficial and important formula in chemistry. It represents how one element or compound changes into another when they react together. Every chemical equation must have a balanced reaction, which means the same amount of atoms of each element present on both sides of the arrow.
Some examples of unbalanced chemical equations are given below.
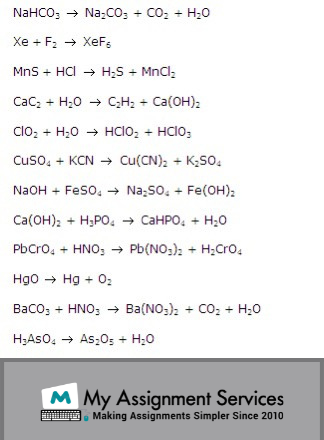
If these were written out in full, then an even number of atoms on both sides would not be shown. So to balance them, we may add coefficients (numbers) in front of the chemical species (particular forms like ions, molecules, radicals) in the equation.
The quantity tells us how many atoms of each element are taking part in the reaction. The coefficients can never be less than 1, and they must be whole numbers (no fractions). The total of the coefficients on one side of an equation should be equal to the sum of the coefficients on the other side. So in our balanced equations above, we have 1 C atom on each side, 2 O atoms on each side, 1 Mn atom on each side, 3 Cr atoms on each side, and finally 1 electron on every side. See the difference between balanced and unbalanced equations? Chemical Equations are used in Chemistry to explain or describe what happens when two or more elements or compounds react together in a chemical reaction.
What are the Different Types of Chemical Equations?
Many types of chemical equations have different reactions. They are as follows: Decomposition reaction, Double replacement reaction, Single replacement reaction, Acid-base neutralisation reaction, Precipitation reaction, and Combustion Reaction. An example is also mentioned in italics and underlined.

Decomposition Equation:-
Decomposition equation is the type of chemical equation in which one compound splits into two or simpler substances when it decomposes. The decomposition reaction itself usually happens very quickly, and so you won't normally see any change during the reaction until after it has finished. For example, hydrogen peroxide (H 2 O 2 ) is decomposed by heat into water(liquid) and oxygen gas(gaseous) when rapidly heated to above 70 °C.
2 H202 --> 2 H20 (liquid) + O2 (gas)
Single Replacement Reaction:
In a single replacement reaction, 1 substance is "substituted" for another in a compound. This usually happens when an element or group of elements in the original compound is replaced by some other elements, ions or atoms. An example of this reaction is magnesium oxide reacting + hydrochloric acid to form magnesium chloride and oxygen gas.
MgO + 2HCl --> MgCl2 + H20
Double Replacement Reaction:
This reaction includes the exchange of two chemical species between ions or molecules in compounds with different compositions. The reaction results in the formation of two new compounds. For E:g, zinc carbonate reacts must interact with ammonium nitrate to form zinc nitrate and ammonium carbonate.
ZnCO3 + (NH4)2SO4 --> Zn(NO3)2 + (NH4)2CO3
Precipitation Reaction:-
In a precipitation reaction, the products of the reaction are a solid and a liquid that separates out from the solution. For example, when hydrogen peroxide is added to potassium iodide solution, it forms an insoluble precipitate of potassium hydroxide and iodine.
6H202 + 2KI --> I2 (insoluble) + 2KOH (soluble) + H20
Acid-Base Neutralisation Reaction:-
An acid-base neutralisation reaction is also known as a neutralisation reaction. In this type of reaction, an acid should react with the base together to form salt & water.
HCl + NaOH --> NaCl + H2O
Combustion Reaction:-
A compound always reacts with oxygen to produce carbon dioxide and water vapour in a combustion reaction.
CH4 + 2O2 --> CO2 + 2H2O
Balanced Chemical Equations:-
As per our five-star rated science assignment help providers, the balanced chemical equation is the equation in which the NO of atoms of each element on both sides is the same. The relative numbers of atoms of each element are proportional on both sides. Balanced equations make it easier to keep track of how many molecules you have at different stages in a chemical reaction. If there is a coefficient in front of a substance in balanced eq, then that substance must be present in equal amounts or produced in equal amounts when the equation is carried out under appropriate conditions.
Here’s How to Balance Chemical Equations
There are various methods to balance chemical equations. They are as follows: By inspection, method Using stoichiometry law.
Method 1:- It is the easiest and very commonly used method to balance chemical equations.
Method 2:- It can be done by following two steps 1) Convert given compound or mole fraction into its equivalent ones .eg if we have N2O5 molecule, then convert it into one molecule of N2O4 and one molecule of O2N2O5 --> 2NO2 + O2 -----> 2NO + 2O2
Method 3:- If there are not any coefficients in front of the substance, then that substance must be either present in equal amounts or must be produced in equal amounts when the equation is carried out under appropriate conditions.
Determining the number of moles existing in a given amount of substance:
To identify the no. of moles present in a given amount of substance, you can use the following relationship.
- Molarity(M) = The number of moles per litre solution of Molar mass.
- (P ) = The pressure exerted by a single gas in an airtight container at equilibrium with other gases and liquids and solid matter Vapour density.
- (V ) =The ratio of the weight of a vapour to the weight of an equal volume of air at the same temperature and pressure.
- Mole fraction (X) = The number of moles of a component divided by the total number of moles in a mixture.
- Molecular Weight (MW) = The sum total of all the atomic weights in a molecule.
- Formula Mass (FM) = The mass of one mole of a compound is expressed in grams.
- Number average Molecular Weight (NAMW) = The weighted average molecular weight, where the weights are proportional to the number of molecules at that point in the distribution.
- Partial Pressure (P ) =The pressure exerted by a single gas in an airtight container at equilibrium with other gases, liquids, and solid matter.
- Vapour Density (V ) = The ratio of the weight of a vapour to the weight of an equal volume of air at the same temperature and pressure.
- Molecular formula (MF) = A chemical symbol with subscripts used to depict molecules. The usual notation for molecular formulas is to enclose the symbols in round brackets and to separate each group within it by a hyphen [-].
For example, NH3, O2.
Mass Percent (X%) = Mass percent is mass per 100 mass unit X%=mass/mass x100 * Molecular formula: C4H10O3 Mass: 164 amu ------> MF: C4H10O3 Formula Weight: 180 amu
Number of atoms in a molecule:
For simple molecules, the no. of atoms in a molecule is (=) to the number of atoms in the formula. For example, in methane (CH4), there are four hydrogen atoms and one CO2. In (H2O), there are 2 hydrogen atoms and one O2 atom.
Percent composition by mass: The % composition by mass is the percentage of each element in a compound by mass. To calculate it, you need to know the atomic weight of each element and the M Weight of the compound.
% Composition by Mass = (Atomic Weight of Element) / (Molecular Weight of Compound) x 100
For example, if you have a compound that is made of two elements, and the atomic weights of those elements are 12 and 16, respectively, the percent composition by mass would be (12/180) x 100 = 6.7%.
Percent composition by atoms: The percent composition by atoms is the percentage of a very element in a compound by atom. To calculate it, you need to know the atomic weights of each element and the M weight of the compound.
% Composition by Atoms = (Atomic Weight of Element) / (Molecular Weight of Compound) x 100
For example, if you have a compound that is made of two elements, and the atomic weights of those elements 12 and 16, respectively, the percent composition by atoms would be (12/16) x 100 = 75%.
Percent composition by the formula: The percent composition by the formula is the percentage of each element in a compound by No atoms. To calculate it, you need to know the compound's molecular weight.
% Composition by Formula = (Atomic Weight of Element)/(Molecular Weight) x 100
For example, if you have a compound that is made of two elements, and the atomic weights are 12 and 16, respectively, but the molecular weight is 180, then the percent composition by formula would be 6.7/180 x 100 = 3.9%.
Percent yield in reaction:- Percent yield in the reaction can be defined as the proportion of product formed compared to the theoretical yield.
For example, you have 12 grams of Be(OH)2, and if 18 grams of it is supposed to be formed in the reaction. The percent yield will be [(18 gms * 100)/12 gms] x 100 = 166%.
Achieve Academic Success With My Assignment Services
It is important to make time for homework and studying as a student. If you need science assignment help for your college coursework, our team of experts can provide the right kind of guidance. Though science may seem like a daunting subject at first glance, we're confident that you will find our science assignment help sessions valuable in understanding what drives human behaviour on an individual level as well as across populations. Fill the form now and we will connect you with the best chemistry experts in Australia.
Related Study Materials
Our Experts can answer your Assignment questions instantly.
Ask Question0 Comment
Get It Done! Today
1,212,718Orders
4.9/5Rating
5,063Experts












Loved reading this Blog? Share your valuable thoughts in the comment section.
Add comment